
Nuclear fission
Two types of uranium occur in nature: the non-fissionable uranium-238 and, to a much smaller extent, the fissionable uranium-235. The numbers 235 and 238 represent the number of nuclear particles that make up the atomic nucleus of this type of uranium. Uranium-238 is stable and cannot split. Uranium-235, however, is unstable. If a freely moving nuclear particle (a neuron) hits this atom, the uranium-235 disintegrates. This releases energy. This is what we call nuclear fission. From a split uranium-235 nucleus, two or three neutrons also shoot out. When these hit other uranium-235 nuclei, they also split again. Thus, a chain reaction is established and the fissile material starts producing heat from these continuous nuclear fissions. In natural uranium, the component fissile uranium-235 is so small (less than 1%) that it is not enough to trigger a nuclear fission reaction. Uranium becomes a good “nuclear fuel” for a nuclear power plant only when it is a mixture of about 96% uranium-238 and 4% uranium-235.

Energy and waste
Nuclear fission produces heat that can be used as a source of energy. It can also be used to produce hydrogen or to make drinking water from seawater. After a few years, the fuel in a reactor is spent. The fragments of a split uranium-235 are radioactive and accumulate in the nuclear fuel until they get in the way of the chain reaction. We call these debris nuclear fission waste. This consists of unstable substances that are lighter than uranium: for example, Strontium-93 or Xenon-140. These newly formed substances are not stable and sooner or later decay into another, stable substance. In the process, radiation is emitted, which is called radioactivity. When all the unstable substances have decayed into a stable end product, there is no radioactivity left. Each radioactive substance decays at a different rate. For some substances this takes a few seconds and for others thousands of years. In the Netherlands, all radioactive waste is safely stored and preserved at COVRA. For more information on the processing and management of radioactive waste, see the topic radioactive waste.

Innovation
Small modular reactors, called Small Modular Reactors or SMRs, which produce carbon-free energy and are highly modular. Nuclear power plants that produce not only electricity but also heat and hydrogen. New medical isotopes that can detect and fight tumors even more precisely. Radioactive generators that make distant space missions to Mars possible. But also ITER, the international cooperation project whose goal is to demonstrate the scientific and technical feasibility of nuclear fusion as a source of energy on Earth. Iter stands for “International Thermonuclear Experimental Reactor.” In short: nuclear technology is in full development. More on this under the topic of innovation.
Image: ISS ©NASA
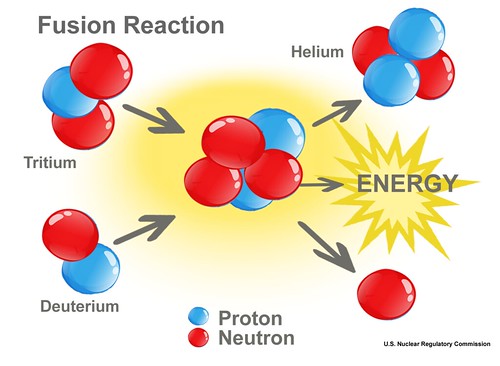
Nuclear fusion
Nuclear fusion is the fusion of the nuclei of several atoms, forming another, heavier nucleus. The lightest atoms in nature, such as hydrogen, are used for nuclear fusion. In the process of fusion, part of the mass is converted into energy, about 0.67% in the case of hydrogen.
Nuclear fusion is not a chain reaction; it does not involve the release of particles that can cause another fusion. The process can only be kept going under extremely high temperature and pressure, much higher than those at the center of the sun. Nuclear fusion, unlike nuclear fission, does not necessarily leave radioactive materials behind as waste. Therefore, scientists are trying to develop nuclear fusion on Earth as a clean and safe source of energy.


