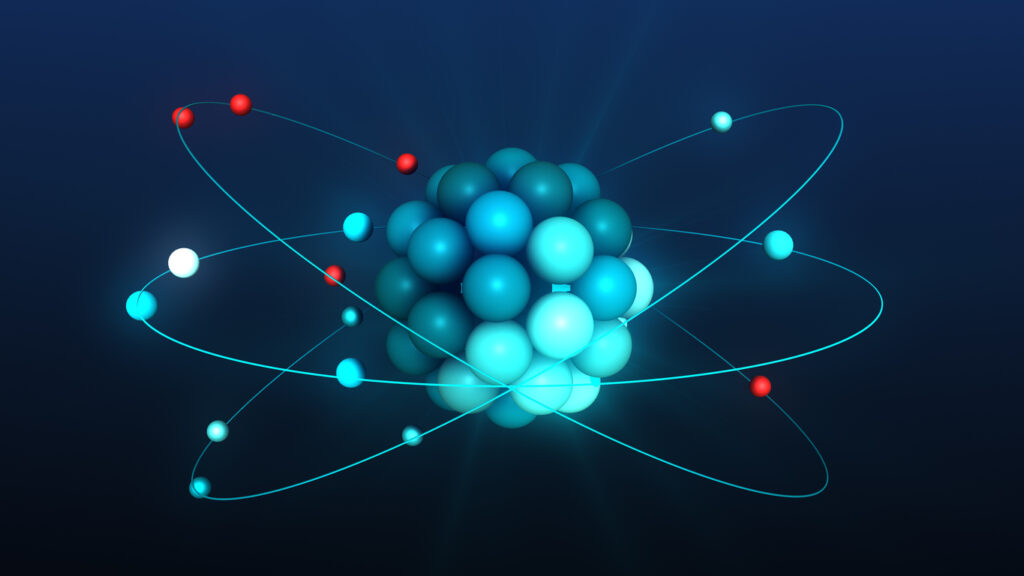
Nuclear fission: the process
Atomic nuclei of the isotope U-235 can be split by nuclear fission into smaller nuclei under emission of radiation, heat and neutrons. These neutrons, in turn, can be captured by other atomic nuclei of U-235, causing new fissions, creating a chain reaction. A controllable chain reaction takes place in a nuclear reactor. To keep the chain reaction going, at least one of the 2-3 neutrons released in a fission must cause another fission. In a stable operating reactor, this is the case and we call the reactor “critical. When more than one neutron remains per fission, you get a “snowball effect. 2 neutrons each produce a fission which both also produce 2 neutrons = 4 neutrons. Those 4 neutrons produce 4 fissions that total 4×2=8 neutrons, etc. When this happens in a reactor, we call the reactor supercritical. This is done in a controlled manner to bring a reactor up to power.
When less than 1 neutron remains per fission, the fission process stops and you refer to it as a subcritical reactor (the reactor is now “off”). In practice, a critical reactor is shut down (made subcritical) by inserting materials that trap neutrons, for example by lowering control rods between the fuel rods. Then there is a shortage of neutrons, causing the chain reaction to stop.
photo: iStock

Enriching uranium
Urenco Netherlands is one of Urenco’s first enrichment facilities, along with the enrichment facility in the United Kingdom, Urenco UK. Since 1973, they have used the centrifuge technology designed and deployed by Urenco to enrich uranium. Urenco’s Dutch enrichment facility is in Almelo. Providing enrichment services to utility customers around the world, they play an essential role in the global nuclear fuel cycle, supporting the production of clean and reliable energy. Currently, two enrichment plants, SP4 and SP5, are in operation. The former plants, SP1, SP2 and SP3, have been completely decommissioned and returned to greenfields. SP5 began operation in 2000 and produces more than 80% of Urenco Netherlands’ total production capacity.
In 1975, Urenco delivered its first SWU contract with pilot plant production at Urenco Netherlands and Urenco UK, and in 1977 our first commercial-scale plant at Urenco Netherlands was officially installed.

Borssele nuclear power plant
EPZ (Elektriciteits-produktiemaatschappij Zuid-Nederland) operates the only working nuclear power plant in the Netherlands: the Borssele nuclear power plant. In this nuclear power plant, uranium atoms are split. EPZ uses the resulting heat to produce steam. This drives a turbine, which drives a generator that generates climate-neutral electricity.The nuclear power plant has a net capacity of 485 MW. That is enough power for a sizable city, including streetcars, trains and a sizable airport. The nuclear power plant has been in operation since 1973 and is adapted to the latest technology every ten years. Thus, the safety of Borssele nuclear power plant is constantly being improved and thus remains safe until 2034. The Dutch government supervises on a daily basis. An independent commission ensures that the nuclear power plant remains in the top 25 percent safest nuclear power plants in the Western world.



