Radiation
Scientists talk about ionizing radiation. In everyday life, we usually talk about radioactive radiation. We often use these terms interchangeably. Ionizing radiation is used in many places in society. In the making of electricity, for medical applications, such as the diagnosis and treatment of diseases and in checking welds for steel structures of windmills, for example. Even nuclear reactors are used for restoring paintings. Ionizing radiation is also present in nature. Our earth and also our own bodies contain radioactive substances, which emit radiation. Everyone is constantly exposed to natural sources of radiation. Only you don’t notice it; humans have no sense of it. Compare it to radio waves, which you can also only perceive if you have a device (radio) for it.
So radiation can only be perceived with instruments. If you carry a special measuring device (a dosimeter), you will see that if you take a plane trip or go skiing high in the mountains, you will receive a much higher radiation dose than during a cycling vacation around the IJsselmeer.
Image: iStock-Nandalal Sarkar

Alpha, beta, gamma
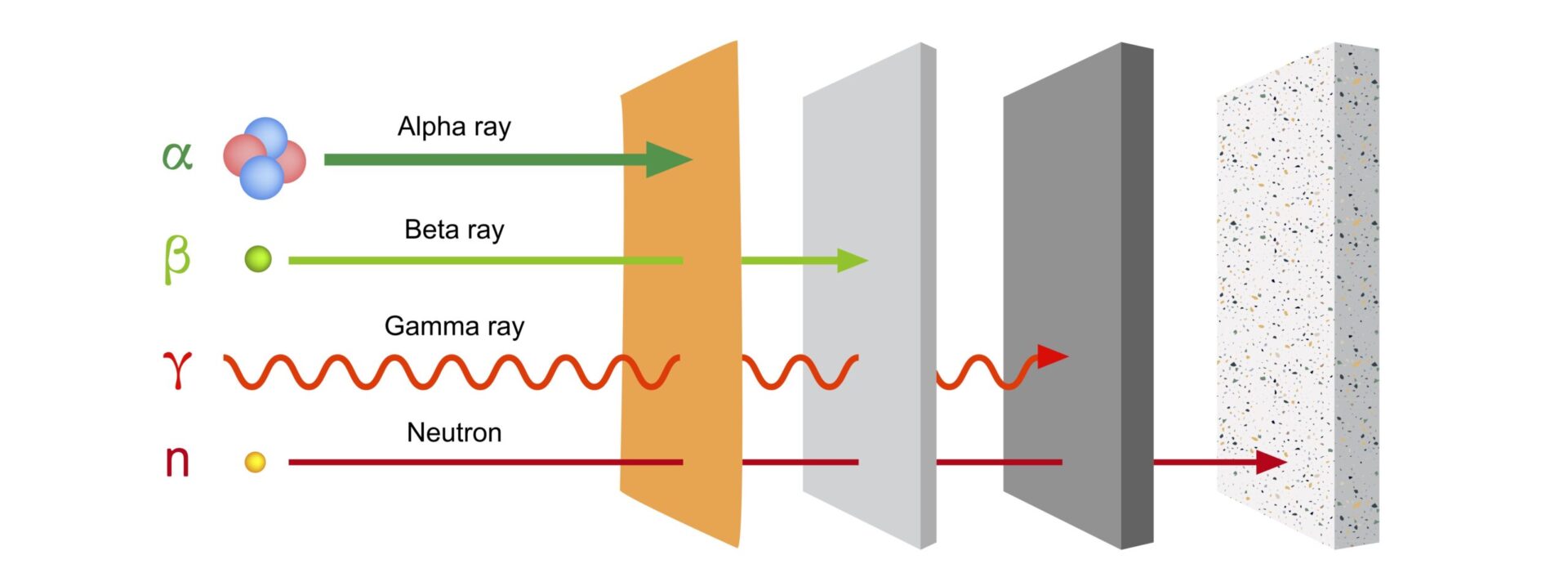
There are three types of radiation: alpha, beta and gamma radiation.
- Alpha radiation consists of relatively large and heavy particles released from a disintegrating atom. We can stop them easily and well. A sheet of paper is sufficient for this purpose. Alpha emitting substances are really only harmful to health if we get them into our bodies through food or breathing. With controls and simple measures such as respiratory protection, you can prevent this.
- Beta radiation consists of lighter particles. They penetrate deeper into matter, but they cannot pass through an aluminum sheet or through three feet of air. We can also protect ourselves well against beta radiation.
- Gamma rays consist of electromagnetic waves and are like light or radio waves. However, gamma rays penetrate further into matter. An example is the X-rays used in hospitals. We can protect ourselves from unwanted gamma radiation by shielding the source with lead or concrete. Many substances emit beta and gamma rays simultaneously.

Half-life
A characteristic of radioactivity is that radiation decreases with time. Nature itself regulates that. The more time passes, the less it radiates. This is due to radioactive decay. The unstable atoms are looking for a new equilibrium. When that equilibrium is reached, the radioactive substance has become stable and no longer emits radiation. How long that takes, we express with half-life. This is the time it takes to lose half of the radioactivity each time. After two half-life periods, the radioactivity is half of half. So that’s a quarter of the initial value. Each radioactive substance has its own fixed half-life. For some substances these are seconds, for others thousands of years. So one radioactive substance is harmless as soon as it is created. Other radioactive material must be stored for many thousands of years before it stops radiating.